Abstract
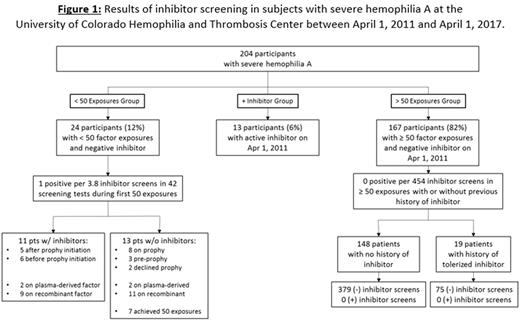
Background : Factor VIII (FVIII) inhibitors comprise the most morbid and expensive complication in severe hemophilia A. Inhibitor risk is known to be highest within the first 50 exposures to FVIII replacement products, with greatest risk during the first 20 exposures. Routine inhibitor screening is typically performed frequently with the first 20 to 50 exposures, but screening after 50 exposures is controversial, given the high cost of testing and unknown benefit. Early inhibitor detection allows effective treatment of bleeding with bypassing agents, and prompt initiation of immune tolerance to eradicate the inhibitor. Here we report inhibitor screening results at the University of Colorado Hemophilia and Thrombosis Center. Methods : This is a retrospective review of inhibitor testing data from study participants with severe hemophilia A enrolled, with Institutional Review Board approved informed consent, in the Hemophilia Prospective Inceptional Cohort Study (HEMO-PICS) from April 1, 2011 through April 1, 2017. A positive inhibitor was defined as modified Nijmegen Bethesda titer ≥0.5 confirmed on 2 separate blood draws. Tolerance was defined as a Bethesda titer <0.5 on two or more samples. The clinical protocol prescribed inhibitor screening in severe hemophilia A patients every 3-5 exposures prior to 50, and annually thereafter in accordance with National Hemophilia Foundation and US Centers for Disease Control recommendations. Results : Of 204 participants with severe hemophilia A in the study cohort, 24 had fewer than 50 factor exposures and were inhibitor negative at the start of the study observation period ("<50 Exposures Group"), 13 participants had a positive inhibitor at the start of the observation period ("+ Inhibitor Group"), and 167 had >50 exposures and a history of either no inhibitor or a tolerized inhibitor (">50 Exposures Group"). Figure 1 shows inhibitor surveillance rates and results. In participants with < 50 exposures, positive inhibitors were detected in 11/42 screening tests, while participants with > 50 exposures had 0 positive inhibitors in 454 screening tests. In the "<50 Exposures Group", 11 of 24 (45%) developed an inhibitor, 7 high titer (peak range 16.7 - 185 BU/mL) and 4 low titer (peak range 0.7 - 1.8 BU/mL). Six participants achieved 50 exposures without inhibitor development during the observation period, while the remaining 7 had fewer than 50 factor exposures at the end of the study period. The mean (+/- standard deviation) age of inhibitor development was 14.6 (+/- 6.2 [range 3-22]) months, after a mean of 12.8 (+/- 7.9 [range 1-29]) factor exposures. Six of those who developed inhibitors did so prior to starting prophylaxis, and the other 5 patients developed inhibitors within 2 months of prophylaxis initiation. Of those who did not develop inhibitors, 8 have been on prophylaxis for an average of 36 (+/- 23.8 [range 14-80]) months, 3 are planning to start prophylaxis, and 2 have declined. Of the 13 participants in the "+ Inhibitor Group", 3 were tolerized during the study. Conclusion : We report a high rate of positive inhibitor surveillance tests in severe hemophilia A patients during the first 50 exposures (1 per 3.8 tests), and very low rates of positive inhibitors in this population following 50 exposures (0/454). While our results reinforce the practice of frequent inhibitor screening in patients with < 50 factor exposures, screening for inhibitors in patients with > 50 exposure days and no change in the bleeding phenotype is unlikely to be clinically relevant and should not be routinely performed.
Warren: CSL Behring Heimburger Award: Research Funding; Bayer Hemophilia Awards Program: Research Funding. Ng: CSL Behring Heimburger Award: Research Funding; Shire: Consultancy. Buckner: Shire: Membership on an entity's Board of Directors or advisory committees; Uniqure: Consultancy; Genentech: Membership on an entity's Board of Directors or advisory committees; Novo Nordisk: Membership on an entity's Board of Directors or advisory committees; CSL Behring: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal